coffee science : everything you need to know about milk -1
being
a barista is a specialised craft revolving around three main products: coffee,
water, and milk. the science behind espresso has already been covered at
length, and there’s been a recent surge of information on water chemistry… yet
milk hasn’t yet received the analysis it deserves.
so
today, we’re going to break down exactly what makes up your milk. this will
help you to make decisions about things like the best temperature for different
drinks, whether to buy full-fat or skimmed, and more.
what
is milk ?
let’s
begin with the basics. this is a bit technical; you may even start to feel like
you’re back in chemistry lessons at school. but don’t worry, because we’re
going to really break it down for you.
milk’s
chemical structure falls under a couple of classifications. scientifically,
it’s referred to as an “emulsified colloid of liquid butterfat globules,
dispersed within a water-based solution”. in english, that’s called “a bunch of
teeny-tiny insoluble protein particles, fat, and other fun stuff, evenly
distributed in water”. the major components are water; protein; fat and sugar
(carbohydrates); and other vitamins, minerals, and salts.
and
in the context of coffee-making, it’s essential for a barista to understand how
these components interact. only this will tell you how they impact on your
beverage.
lactose,
aka how much should you heat that milk ?
lactose
is a diassacharide (two sugars) made up of galactose and glucose. it kind of
looks like this:
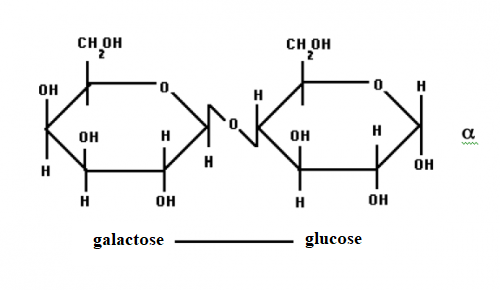 |
| credit: uoguelph.ca |
yet
while lactose is classified as a sugar, it’s only 16/100 as sweet as regular
sugar (sucrose). it also reacts rather interestingly to the application of heat
and hydrolysis (using water to unbind the chemicals). you see, steaming milk
adds both heat and water to the mix. this is why a steamed milk tastes sweeter
than, say, milk heated on a stovetop or in a microwave.
the
optimal temperature for steaming milk is a hot-topic amongst baristas, but the
core of this debate is one question: “at what temperature does milk taste the
sweetest?”
but
the answer is really much less dependant on the temperature than it is on the
lactose content! as much of a no-brainer this is, a milk with a higher lactose
content will always taste sweeter, regardless of what temperature you’re
steaming it at. conversely, a milk with a low lactose content (under 3%) won’t
get that mellow, much-desired sweetness – no matter what you do to it. as a
gauge, most commercial brands of milk you find in cafés have a lactose content
of anything between 4-5%.
so
why does hot milk taste sweeter? because the human tongue is naturally more
sensitive to sweetness when things are hotter. this explains why a cold soda
tastes refreshing and balanced, but a warm one is cloyingly sweet.
something
interesting to note, though, is that it does affect the proteins – which is why
“burnt” milk is thinner. “burning” milk is a no-no because of how it affects
the consistency of the milk, not because it has any effect whatsoever on the
sweetness of the milk.
another
fun fact: in the culinary world, there are several classic french recipes,
bechamel sauce being the best example, that require you to burn the milk. this
makes sense because, with the thinner consistency of burnt milk, incorporating
it into sauces is easier and it also reduces the chances of lumps forming.
there
are several brands of lactose-free milks on the market that extract lactose
with an enzyme called lactase, leaving a residue of the (much, much sweeter)
glucose and galactose. as a result, lactose-free milk often tastes confusingly
sugar-sweet.
protein,
aka how good will my foam be ?
if
fat and lactose were your friends, protein would be that third-wheeler that you
never really invited to birthday parties. i think it’s partly because talking
about protein isn’t particularly exciting, but also because it’s flavourless
and has less of an obvious impact on us.
that
said, protein, in the context of steaming and frothing, is probably the most
crucial component of milk. yes, i mean it.
there
are two main proteins in milk: caseins (80%) and amino acids, also called whey
proteins (20%).
but what’s interesting to know is that milk proteins are partially hydrophobic. okay, i get it, you’re staring at me pretty blankly. but this basically means that one end of the chain wants to stick to water but the other end of it wants to get the hell away. this is what actually gives milk that opaque, white colour. and, most importantly, this property is responsible for building the foam structure.
but what’s interesting to know is that milk proteins are partially hydrophobic. okay, i get it, you’re staring at me pretty blankly. but this basically means that one end of the chain wants to stick to water but the other end of it wants to get the hell away. this is what actually gives milk that opaque, white colour. and, most importantly, this property is responsible for building the foam structure.
let
me explain how this works. imagine a giant ball-pit, the kind with the
multicoloured plastic balls. now imagine, for a second, that all these balls
were joined up with fishing line thread or something, forming a rope of balls,
so to speak. these ropes are all tangled up and mixed together, so you can still
jump in the pit and swim around in it without any obstruction.
suddenly,
steam (aka heat and water and air) is added into the pit! argh ! chaos !
the
heat is breaking down the chains into smaller chains; some of them are even unravelling. and to make things even weirder, the balls are all repelling the
water! they wrap around the air bubbles, since that’s the only thing they can
kind of cling on to. suddenly, some kind of grid-like structure is formed… it
kind of looks like foam !
 |
a
much looser foam for a better visual understanding.
credit:
modernistcuisine.com
|
what
all of this means is that, if your milk has more protein, it will have a more
stable foam structure.
naturally
produced as-is milk usually has a protein content of 3.3%, but in most
commercial milks, more protein is added to make the heat-related process of
homogenisation easier. so your milk could probably contain anything between
3.6-4.1%.
article written by christine s.
edited by t. newton.
feature photo credit: chris pelliccione




Yorumlar
Yorum Gönder